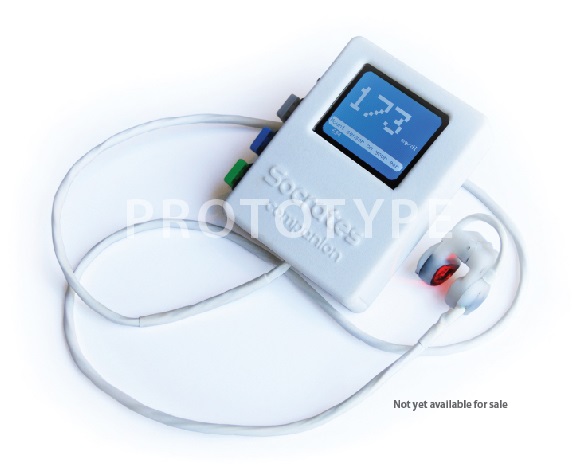
Back in September, D Healthcare Daily brought you the story of Scott Smith, a healthcare entrepreneur who risked everything, quitting his job and throwing his efforts behind Companion, a noninvasive glucose monitor. His mission and company name—Socrates Health Solutions—were inspired by Smith’s father, a diabetic linguistics professor and philosophy buff who was fond of quoting Socrates and what is known as the Socratic paradox: “I know one thing: that I know nothing.”
And now, to fund a company named after a 5th century B.C. philosopher, Smith has taken a 21st century approach. This week he launched an IndieGoGo site, with the hope of raising $125,000. That money will go toward producing Companion.
“Though Companion is a working prototype today, it is NOT FOR SALE and will not be available to the general public until it passes FDA approval,” he wrote. “That is why we need your help. The funds received from this project will go to support the FDA process, clinical trials, legal expenses and operational requirements needed to pass FDA approval.”
Smith developed a new way to determine glucose levels in the bloodstream without the painful and costly “prick” method available today. By attaching the Companion sensor to the ear, the user can get an accurate blood glucose reading in seconds.
So far, $705 has been raised.
Smith said he hopes to complete FDA clinical trials in 2014 and begin to sell Companion in early 2015. He is seeking to raise $6 million for manufacturing and regulatory hurdles, and is talking to three companies across North Texas and California to help fund the effort and become equity partners.
Once Smith raises the capital, he anticipates his other six executive-team members will quit their day jobs as well; five of the seven worked together in Smith’s previous entrepreneurial ventures. Socrates has hired a consultant who has ushered 18 products through the FDA-approval process, including two other blood glucose monitors, and a local law firm to protect the product’s patent.
Check out the video below for more information.





